Generally, humane endpointsHumane endpoint The moment in the experiment at which pain and/or distress experienced by the investigational animal is ended or alleviated by either killing the animal humanely or by discontinuing the procedure. can be based on different categorizing indicators like:
- clinical signs (e.g., tumorTumor Neoplasm, new growth. Is predicated upon autonomous growth of cells or tissues into benign or malignant tumors. formation);
- pathophysiological changes (e.g., drop in body temperature);
- behavioural changes (e.g., stereotypic behaviourStereotypic behaviour A phenomenon that appears as a response to chronic stress. It is manifested as a set repeated pattern of seemingly purposeless activity.);
- biochemical changes (e.g., ketonuria);
- hormonal changes (e.g., prolactin).
| Clinical/ behavioural | Pathophysiological | Biochemical |
| Activity | Respiration rate | Acute phase proteins |
| Posture | Blood count | Catecholamines |
| Aggression | Weight loss | CorticosteroidsCorticosteroids Steroid hormones produced by the adrenal cortex. |
| Response to handlingHandling Manipulating the animal, picking it up, holding it, and restraining it. | Heart rate | Glucagon |
| GroomingGrooming The behavior animals engage in to keep clean. Grooming removes dirt from fur and body openings. Absence of this behavior indicates reduced well-being. | DehydrationDehydration “Drying out” because of reduced fluid intake or excessive fluid loss. The degree of dehydration can be determined by gently pinching and twisting the skin and releasing it, allowing the skin to fold and flatten (the turgor test). | Insulin |
| VocalisationVocalisation Sounds produced by animals that may or may not (ultrasound, infrasound) be audible to humans. The rat associates 20 kHz sounds with fear or pain and 50 kHz sounds with pleasant situations. | Anury | Prolactin |
New developments in the field of humane endpoints
New developments in the field of humane endpoints focus on achieving maximal reduction of painPain The negative sensory or emotional experience that indicates awareness in the animal of injury or the threat of injury to the body. This negative experience induces changes in an animal’s behavior and physiology, intended to limit the effect of, or avoid the injury, to reduce the chances of repeated injury and to promote recovery. and/or distressDistress It indicates a disadvantageous environment in which the animal is no longer able to adapt in a biologically successful manner to the stressors to which it is exposed. One speaks of distress when the level or the duration of stress are such that significant changes in biological function are required to survive. and, if possible, to eliminate them completely. Generally speaking, the developments can be grouped into one of two categories:
1. Preclinical humane endpoints
Preclinical humane endpoints are endpoints that can be identified before the onset of pain or distress.
For example, think of the use of non-invasive measuring technologies such as bioluminescence. This molecular biology technique involves labeling of virulent micro-organisms or tumor cells with the luciferase gene. The labeled pathogen or tumor cell can then be followed in a non-invasive manner. This technology can be used to gauge the growth or dissemination of the pathogen or the tumor cell (e.g., in metastatic disease), allowing the animal to be removed from the experiment at an early stage—before clinical signs appear.
Another example of a preclinical endpoint may be found in the use of molecular biology techniques such as transcriptomics (expression profiling). This means that in carcinogenicity studies, the endpoint may be the up- or down-regulation of the genes associated with tumor growth, rather than the appearance of the tumor itself.
2. Non-clinical humane endpoints
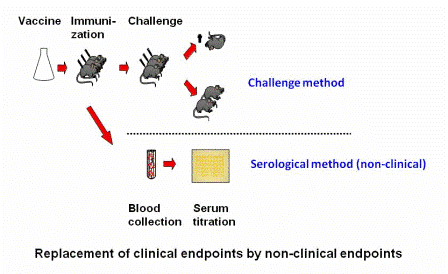
With non-clinical humane endpoints, the procedure in the experiment that will ultimately lead to clinical signs is replaced by a procedure that will not result in clinical signs. A clear example is to be seen in the efficacy study for a newly produced batch of vaccine. Until recently, this type of study to determine the degree of protection afforded by a vaccine required challenging vaccinated animals several weeks post immunization with an injection of the virulent micro-organism (or its toxin). The degree to which the animals were protected was taken as the measure of efficacy of the vaccine.
Nowadays, for several types of vaccines, the illness-inducing challengeChallenge Experimental provocation (induction) of an illness, for instance an experimental infection, in order to determine if an investigational therapy or prophylaxis is effective. Challenge models are common in the study of infectious diseases and for testing medications and vaccines. procedure may be replaced with a blood test in which the degree of protection is determined by in vitro antibody titration (i.e., by demonstrating pathogen or toxin neutralization in tissue culture or in an ELISA assay).